Research yields ideas for novel methods in treatment of sports injuries, heart attacks and other musculoskeletal disorders such as muscular dystrophies
New research in the School of Biological Sciences at UC Irvine is raising hope for potential new methods to help the millions of people who suffer from painful tendon injuries.
Post-doctoral fellow Arul Subramanian and Thomas Schilling, professor of developmental & cell biology, have recently published a study in the open-access journal eLIFE detailing their discovery of a special scaffolding protein that maintains configuration of attachments between tendons and muscles in animals.
Their work is yielding ideas for novel treatment methods for sports injuries, heart attacks and other musculoskeletal disorders such as muscular dystrophies.
Tendons are collagen-rich tissue that connect muscles with bones and are often the cause of debilitating sports injuries and work accidents as well as genetic or aging-associated degenerative conditions.
While most treatments of tendon injuries focus on surgical intervention or the use of stem cells, these methods are generally not very effective and require a prolonged period of recuperation, rehabilitation and pain-relieving medication. Understanding the formation of tendons, in addition to understanding the mechanisms behind the organization and assembly of tendon matrices, will help develop effective methods of treatment for tendon injuries and diseases.
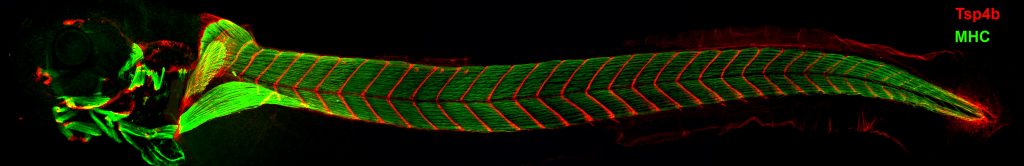
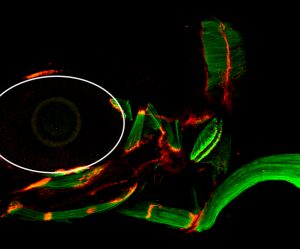
In their study, Subramanian and Schilling screened for proteins involved in the formation and control of tendon matrix assembly in zebrafish and identified a novel protein – Thrombospondin-4 (Tsp-4) – as an essential scaffold for formation and maintenance of the tendon matrix assembly. Loss of Tsp-4 caused weakening of muscle attachments that detached readily upon increased contractile force in developing zebrafish embryos.
The researchers found that Tsp-4 is essential for activation of integrin receptors on the muscle surface for proper muscle attachment and maturation and also for localization of laminin at the muscle-tendon junction.
Tsp-4 functions as a pentamer, and this distinctive pentameric arrangement facilitates the specialized assembly of laminin and collagen proteins in the tendon. Subramanian and Schilling also discovered that the human Tsp4 protein was able to rescue the muscle detachment in zebrafish embryos that were deficient for zebrafish Tsp4 and strengthened muscle attachments from increased stress of muscle contraction.
Human Tsp4 levels are elevated in certain forms of muscular dystrophies and mutations in Tsp4 are correlated with myocardial infarction. The unique function of Tsp4 in serving as a scaffold for assembly of stress-bearing and force-transmitting matrix proteins is important for both skeletal muscles and cardiac muscles due to the similar level of stress and force associated. The conserved function of Tsp4 in humans and zebrafish provides new avenues towards developing better approaches in treating injuries associated with the connective tissue of musculoskeletal and cardiac systems.
The eLIFE study can be accessed at: http://elifesciences.org/content/3/e02372. eLIFE is an online journal highlighting outstanding research in the life sciences and biomedicine. The National Institutes of Health provided funding support (grants R21 AR62792 and R01 DE13828).
Three-day- old zebrafish larvae showing muscles stained with muscle-specific myosin antibody (green), and tendons stained with thrombospondin-4b antibody (red). Tsp4b is localized at all muscle attachment sites in the larva.
If you’d like to learn more about how you can support the faculty and research at the Biological Sciences School, please contact Andrew DiNuzzo at 949.824.2734 or adinuzzo@uci.edu.