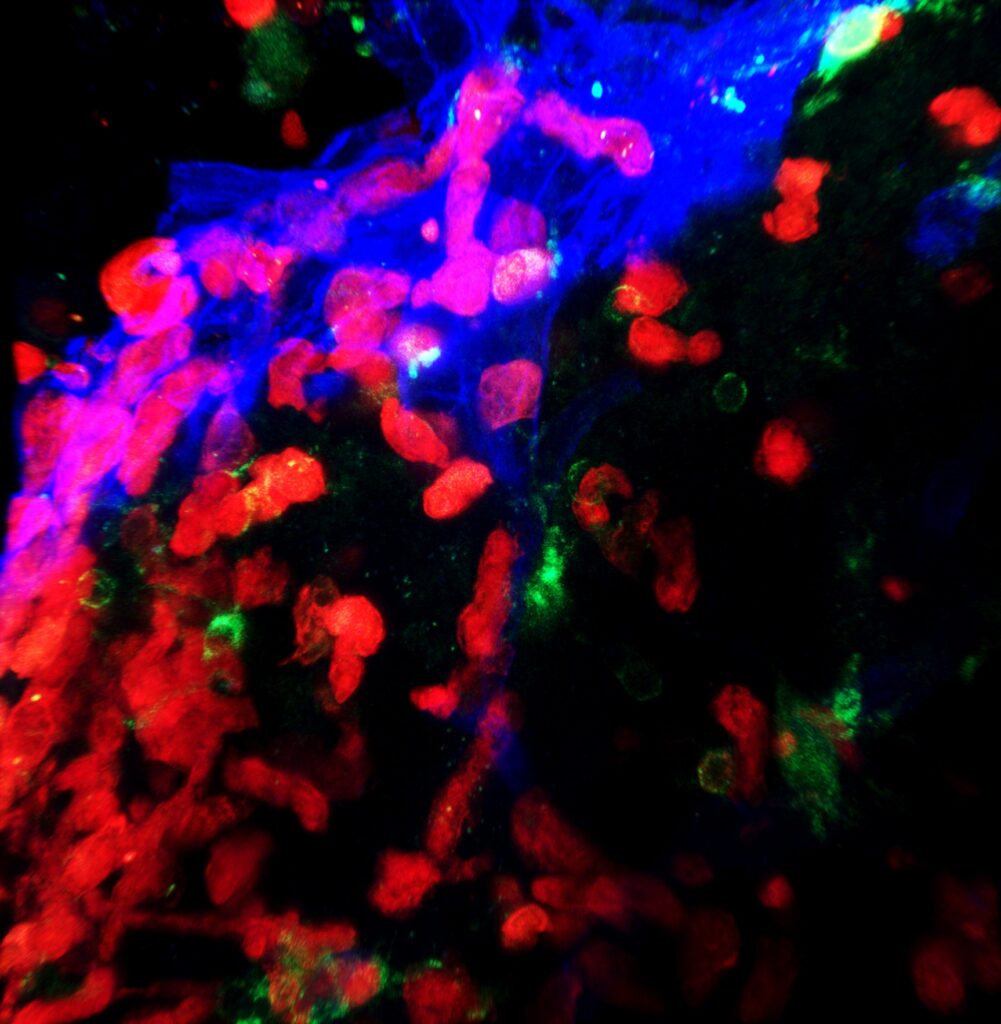
Photo credit: Rocio Barahona, Lindsay Hohsfield, and
Kim Green.
Irvine, Calif., June 3, 2025 — Scientists at the University of California, Irvine have uncovered a vital link in the brain’s communication with the immune system — one that has been hiding in plain sight. In a new study published in Neuron, researchers from the Charlie Dunlop School of Biological Sciences reveal that a known but underappreciated brain structure, the velum interpositum, serves as a key route for immune cells to enter the brain. This discovery could reframe how scientists approach the treatment of diseases that involve the immune system’s interaction with the brain, such as multiple sclerosis, Alzheimer’s disease and other neurodegenerative disorders.
While the velum interpositum — a thin, vascularized membrane located beneath the hippocampus — has been documented in anatomical literature for decades, its role in immune cell trafficking was unknown until now. The UC Irvine team demonstrated that this structure acts as a specialized gateway through which myeloid cells, a class of immune cells, can move from the blood through a specialized structure that resembles the meninges (the brain’s protective membranes) into the brain parenchyma, the functional tissue involved in processing information and coordinating body functions.
“This study identifies a novel structure, the velum interpositum, as a gateway for immune cells to enter the brain,” said Kim Green, senior author of the study and professor of neurobiology and behavior at the Dunlop School. “We were able to track cells traveling from the blood into the meninges, and from there deep into the critical regions of the brain, via the velum interpositum. These findings provide important insights into our understanding of how the immune system interacts with the brain in various neurological diseases, which could lead to the development of new therapeutic approaches.”
One of the most significant hurdles in this study was figuring out how to observe immune cells entering the brain — something current technology can’t capture directly. “Our biggest challenge was figuring out how to actually see immune cells entering the brain,” said Lindsay Hohsfield, the study’s first author and associate researcher in the Green Lab. “So, we had to get creative. We used advanced 3D imaging techniques to get a full picture of the brain and developed innovative ways to track specific immune cells. Using a combination of methods including bone marrow transplants, microglial depletion and careful inspection of the brain allowed us to identify the unique pathway and cells that enter the brain.”
In healthy conditions, the immune system performs essential roles in brain maintenance and protection. However, in certain disease states, an overactive or misdirected immune response can accelerate damage to neurons and brain structures. By identifying the velum interpositum as a focal point of immune cell entry, the researchers have provided a new target for therapies that could modulate or restrict immune activity in the brain — potentially reducing disease severity or progression.
The study also introduced a new technique to selectively eliminate macrophages (a type of myeloid cell) at the interface between the blood and brain without affecting other cells. “We developed a novel method to target and eliminate macrophages that reside at specific areas where the periphery and blood meet,” Hohsfield said. “This work opens the door to a completely new avenue of research and brain therapies that could help address problems with communication between the immune system and the central nervous system.”
The implications of these findings reach far beyond anatomy. By uncovering how immune cells access the brain through a previously unrecognized route, this research opens new possibilities for treating neurodegenerative disorders where the immune system plays a harmful role. It also highlights the continued importance of foundational scientific discovery, reminding us that even long-known structures can still hold powerful new insights into how the brain works — and how we might better protect it.
About the University of California, Irvine Charlie Dunlop School of Biological Sciences:
Recognized for its pioneering research and academic excellence, the Charlie Dunlop School of Biological Sciences plays a crucial role in the university’s status among the nation’s top 10 public universities, as ranked by U.S. News & World Report. It offers a broad spectrum of degree programs in the biological sciences, fostering innovation and preparing students for leadership in research, education, medicine and industry. Nestled in a globally acclaimed and economically vibrant community, the school contributes to the university’s impact as Orange County’s largest employer and a significant economic contributor. Through its commitment to exploring life’s complexities, the Dunlop School embodies the UC Irvine legacy of innovation and societal impact. For more on the Charlie Dunlop School of Biological Sciences, visit https://www.bio.uci.edu/.
